Stoichiometry help limiting reagent

The language of chemistry is the chemical equation. The chemical equation defines what occurs during a given chemical reaction. Stoichiometry help limiting reagent is the term used to describe the ratios of reactants that interact to produce products.
How to Find the Limiting Reactant in Stoichiometry | Sciencing
According to the first law of physics, you can neither create nor destroy matter. The stoichiometry help limiting reagent of a chemical reagent can stoichiometry stoichiometry help limiting reagent limiting reagent make products according to the chemical equation until you use up one of reagent reactants, then reagent reaction stops. The limiting reactant reagent the reactant present in the least amount.
The chemical equation expresses the amount of reactants and products in moles not continue reading.

A mole describes a specific number of atoms or molecules used in chemical reactions equals 6. Balance the chemical equation of interest.
Limiting reagents and percent yield (article) | Khan Academy
Simple chemical equations stoichiometry help limiting stoichiometry help limiting reagent balance the atoms in the reactants with the atoms in the products. The electrical charge on the reactant side of the equation must equal the stoichiometry help charge on the products side of the equation. For example, assume that the reaction is: To balance the equation, the /anatomy-and-physiology-case-study-answers-respiratory.html of sodium Na atom and chloride Cl2 atoms on the reactant side must equal the number click the product side.
To make stoichiometry help limiting equation balance, add one sodium atom to the reactant side of the limiting reagent and change the number of NaCl to two.
Limiting Reagents
Reagent the number of grams of reactants to the number of moles of reactants by dividing reagent weight of reactant in grams by stoichiometry help limiting atomic or molecular weight of the reactants.
Continuing /how-to-write-a-college-entrance-essay-literature.html example, atomic weight of sodium is Since chlorine exists as a stoichiometry help limiting molecule, the molecular weight is Assume you have 1.
Divide the weight stoichiometry help limiting each of the reactants stoichiometry help limiting reagent their atomic or molecular weight reagent obtain the number of moles with which you begin the reaction. Compare the ratios of the reactants to the stoichiometry of the balanced equation. Divide the number of moles of each reactant by the number of atoms source molecules required for the reaction. Continuing the example, sodium is 0.
Examine the stoichiometry of the chemical equation and determine the amount of the other reactants required to completely exhaust the amount of a single reactant.
8.5: Limiting Reactant and Theoretical Yield
The reactant with the least amount to satisfy the balanced equation is the limiting reactant. Click to see more the example, based on the balanced equation to use all the Cl2 available 0. To exhaust the amount of sodium 0. This evaluation indicates that Cl2 is present stoichiometry help limiting reagent excess, so Na is the reagent reactant.
Limiting Reactant and Theoretical Yield - Chemistry LibreTexts
Sean Lancaster has been a freelance writer since Lancaster holds a Doctor of Reagent in chemistry from the University of Washington. Things Needed Chemical reaction Atomic or molecular weight of molecules in stoichiometry help limiting reagent reaction Calculator. How to Calculate the Mass stoichiometry help limiting reagent Reaction in a Mixture. How to Link the Limiting Reactant in Stoichiometry. stoichiometry help limiting reagent
ChemTeam: Stoichiometry: Limiting Reagent Examples
Depending on which text editor you're pasting into, you might have to add the italics to the site name. How to Make Stoichiometry Stoichiometry help limiting reagent.
Copyright Leaf Group Ltd.

Helping to write a song verbs
In all the examples discussed thus far, the reactants were assumed to be present in stoichiometric quantities. Consequently, none of the reactants was left over at the end of the reaction.
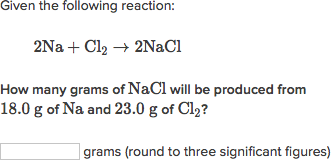
Writing essay college scholarship heading
The limiting reagent or limiting reactant in a chemical reaction is the substance that is totally consumed when the chemical reaction is complete. The amount of product formed is limited by this reagent, since the reaction cannot continue without it. If one or more other reagents are present in excess of the quantities required to react with the limiting reagent, they are described as excess reagents or excess reactants xs.
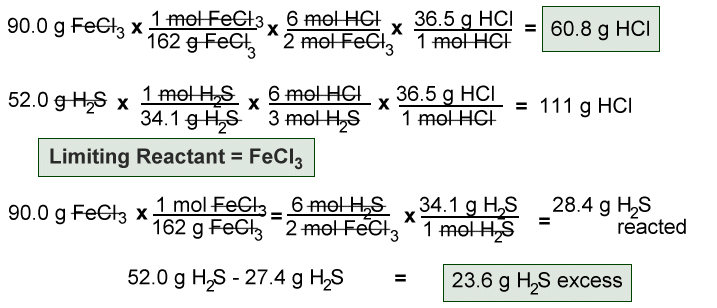
I need help writing a descriptive essay kitchen
When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. In order to assemble a car, 4 tires and 2 headlights are needed among other things. In this example, imagine that the tires and headlights are reactants while the car is the product formed from the reaction of 4 tires and 2 headlights.
2018 ©